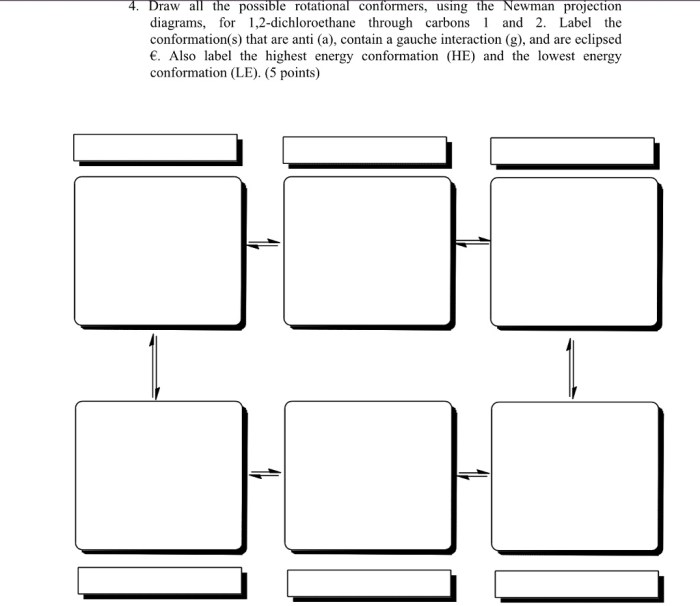
Newman projection 1 2 dichloroethane – Newman projection 1,2-dichloroethane, a cornerstone of conformational analysis, offers a unique perspective into the three-dimensional arrangement of molecules. This detailed exploration delves into the intricacies of Newman projections, unraveling their significance in understanding molecular properties and reactivity.
Delving into the concept of Newman projections, we elucidate their ability to represent the spatial orientation of atoms and functional groups around a specific carbon-carbon bond. The Newman projection of 1,2-dichloroethane serves as a prime example, showcasing the distinct conformations adopted by this molecule.
Newman Projection of 1,2-Dichloroethane
A Newman projection is a two-dimensional representation of a molecule that shows the relative positions of the atoms along a specific bond. It is useful for visualizing the different conformations of a molecule and understanding their relative stability.
To draw a Newman projection of 1,2-dichloroethane, we first choose a C-C bond as the reference bond. We then place one of the carbon atoms at the center of the projection and draw the other carbon atom behind it. The front carbon atom is represented by a dot, while the back carbon atom is represented by a circle.
The substituents on the carbon atoms are then drawn as lines. The lines that are closer to the viewer are drawn as solid lines, while the lines that are farther away are drawn as dashed lines.
The Newman projection of 1,2-dichloroethane is shown below:

Conformational Analysis
Conformational analysis is the study of the different conformations of a molecule and their relative stability. The conformation of a molecule is defined by the relative positions of its atoms in space.
1,2-Dichloroethane has two different conformations:
- The eclipsed conformation, in which the two chlorine atoms are directly opposite each other.
- The staggered conformation, in which the two chlorine atoms are as far apart as possible.
The staggered conformation is more stable than the eclipsed conformation because it has less steric hindrance. Steric hindrance is the repulsion between electron clouds of atoms or groups of atoms that are close together.
Energy Barriers, Newman projection 1 2 dichloroethane
The energy barrier for rotation around the C-C bond in 1,2-dichloroethane is the amount of energy that must be overcome in order to convert the molecule from one conformation to another.
The energy barrier for rotation around the C-C bond in 1,2-dichloroethane is relatively low, which means that the molecule can easily convert between the eclipsed and staggered conformations.
Applications of Newman Projections
Newman projections are used in a variety of applications in chemistry, including:
- Predicting molecular properties and reactivity
- Understanding the mechanisms of chemical reactions
- Designing new molecules with specific properties
FAQ Section: Newman Projection 1 2 Dichloroethane
What is the significance of the energy barrier in conformational analysis?
The energy barrier determines the rate at which molecules can interconvert between different conformations. Higher energy barriers hinder conformational changes, leading to slower reaction rates and increased molecular stability.
How can Newman projections be used to predict molecular reactivity?
Newman projections provide insights into the steric interactions between atoms and functional groups. By identifying the most stable conformation, chemists can predict the preferred reaction pathways and regioselectivity of chemical reactions.