In the realm of chemistry, understanding the arrangement of atoms and ions based on their radii holds immense significance. This concept, known as arrange the atom and ions according to radius, provides crucial insights into the fundamental properties and behaviors of elements and compounds.
By delving into the factors that influence atomic and ionic radii, we can unravel the intricate relationships that govern the chemical world.
The concept of atomic and ionic radii serves as a cornerstone in various fields of chemistry, including inorganic chemistry, materials science, and biochemistry. It plays a pivotal role in determining the stability of chemical bonds, predicting crystal structures, and understanding the reactivity of molecules.
This article embarks on a comprehensive exploration of arrange the atom and ions according to radius, shedding light on its definition, measurement techniques, and applications.
Atomic Radius
Atomic radius is the distance from the nucleus to the outermost electron shell of an atom. It is typically measured in picometers (pm), where 1 pm is equal to 10 -12meters.
Atomic radius can be measured using various techniques, such as X-ray crystallography and electron diffraction. The atomic radius of an element can vary depending on its atomic number, the number of electrons in its outermost shell, and its chemical environment.
Examples of Elements with Different Atomic Radii
- Hydrogen: 53 pm
- Carbon: 70 pm
- Nitrogen: 75 pm
- Oxygen: 73 pm
- Sodium: 190 pm
- Potassium: 235 pm
Ionic Radius
Ionic radius is the distance from the nucleus to the outermost electron shell of an ion. It is typically measured in picometers (pm), where 1 pm is equal to 10 -12meters.
Ionic radius can be measured using various techniques, such as X-ray crystallography and electron diffraction. The ionic radius of an ion can vary depending on its charge, the number of electrons in its outermost shell, and its chemical environment.
Examples of Ions with Different Ionic Radii, Arrange the atom and ions according to radius
- Na +: 102 pm
- K +: 138 pm
- Cl –: 181 pm
- Br –: 196 pm
- I –: 220 pm
Factors Affecting Atomic and Ionic Radii

Factors Affecting Atomic Radius
- Atomic number: As the atomic number increases, the number of electrons in the atom increases, which leads to an increase in the atomic radius.
- Electron configuration: The electron configuration of an atom can affect its atomic radius. Atoms with more electrons in their outermost shell tend to have larger atomic radii.
- Shielding effect: The shielding effect is the ability of inner electrons to shield the outermost electrons from the attraction of the nucleus. The more inner electrons an atom has, the greater the shielding effect, and the larger the atomic radius.
Factors Affecting Ionic Radius
- Charge: The charge of an ion affects its ionic radius. Ions with a higher charge tend to have smaller ionic radii.
- Electron configuration: The electron configuration of an ion can affect its ionic radius. Ions with more electrons in their outermost shell tend to have larger ionic radii.
- Lanthanide contraction: The lanthanide contraction is the decrease in ionic radius across the lanthanide series. This contraction is due to the poor shielding effect of the 4f electrons.
Trends in Atomic and Ionic Radii
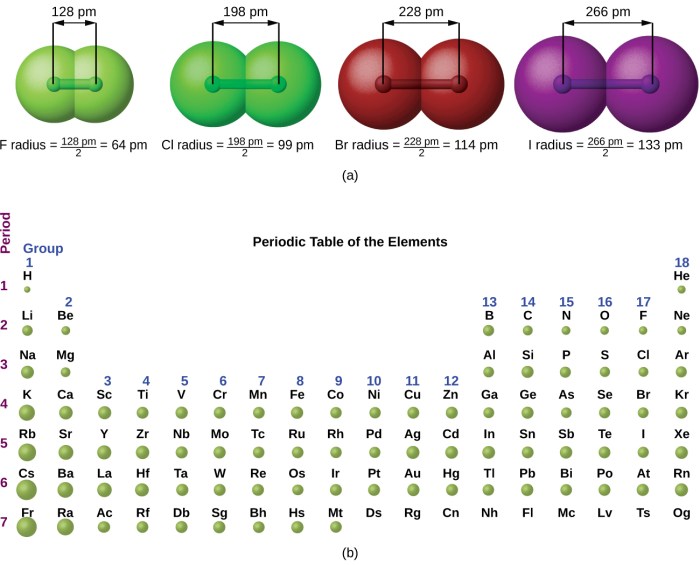
Trends in Atomic Radii Across the Periodic Table
- Atomic radius generally increases from right to left across a period.
- Atomic radius generally increases from top to bottom within a group.
Trends in Ionic Radii Across the Periodic Table
- Ionic radius generally decreases from left to right across a period.
- Ionic radius generally increases from top to bottom within a group.
Reasons for These Trends
The trends in atomic and ionic radii can be explained by the factors that affect these radii. For example, the increase in atomic radius from right to left across a period is due to the increase in the number of electron shells.
Applications of Atomic and Ionic Radii
Atomic and ionic radii are important in understanding the structure and properties of materials. For example, the atomic radius of an element can be used to predict its melting point and boiling point.
Ionic radii are important in understanding the formation of ionic compounds. For example, the ionic radius of an ion can be used to predict the stability of an ionic compound.
Examples of How Atomic and Ionic Radii are Used in Practice
- Atomic radii are used to design new materials with specific properties.
- Ionic radii are used to predict the solubility of ionic compounds.
Helpful Answers: Arrange The Atom And Ions According To Radius
What is atomic radius?
Atomic radius refers to the distance from the nucleus of an atom to the outermost electron shell.
How is atomic radius measured?
Atomic radius is typically measured in picometers (pm) or angstroms (Å) using techniques such as X-ray crystallography and electron diffraction.
What factors affect atomic radius?
Factors influencing atomic radius include the number of electrons, nuclear charge, and shielding effect.
What is ionic radius?
Ionic radius refers to the distance from the nucleus of an ion to the outermost electron shell.
How is ionic radius measured?
Ionic radius is measured using similar techniques as atomic radius, but it considers the changes in electron configuration upon ion formation.
What factors affect ionic radius?
Factors affecting ionic radius include the charge of the ion, the size of the parent atom, and the electronegativity of the ion.