Diagrama del ciclo del nitrógeno – The nitrogen cycle diagram provides a comprehensive visual representation of the intricate processes that govern the transformation and circulation of nitrogen within the Earth’s ecosystems. This cycle encompasses a series of interconnected steps, each playing a crucial role in maintaining the delicate balance of life on our planet.
Nitrogen, an essential element for all living organisms, undergoes various chemical transformations as it cycles through the atmosphere, soil, and water. Understanding the nitrogen cycle diagram is vital for grasping the significance of these processes and their implications for the health of our environment.
Nitrogen Cycle Diagram Overview
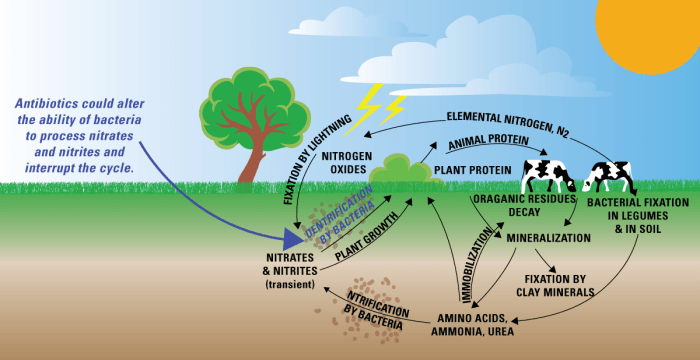
The nitrogen cycle is a fundamental biogeochemical process that ensures the continuous availability of nitrogen in various forms, supporting life on Earth. It involves a series of transformations and exchanges between the atmosphere, soil, plants, animals, and microorganisms.
The nitrogen cycle encompasses several key processes:
- Nitrogen Fixation:Atmospheric nitrogen (N 2) is converted into ammonia (NH 3) by nitrogen-fixing bacteria and certain industrial processes.
- Nitrification:Ammonia is oxidized to nitrite (NO 2–) and nitrate (NO 3–) by nitrifying bacteria.
- Assimilation:Plants absorb nitrate and ammonia from the soil, converting them into organic nitrogen compounds, such as proteins and nucleic acids.
- Ammonification:Organic nitrogen in dead plants and animals is decomposed by bacteria, releasing ammonia into the soil.
- Nitrous Oxide Production:Nitrous oxide (N 2O) is produced as a byproduct of nitrification and denitrification.
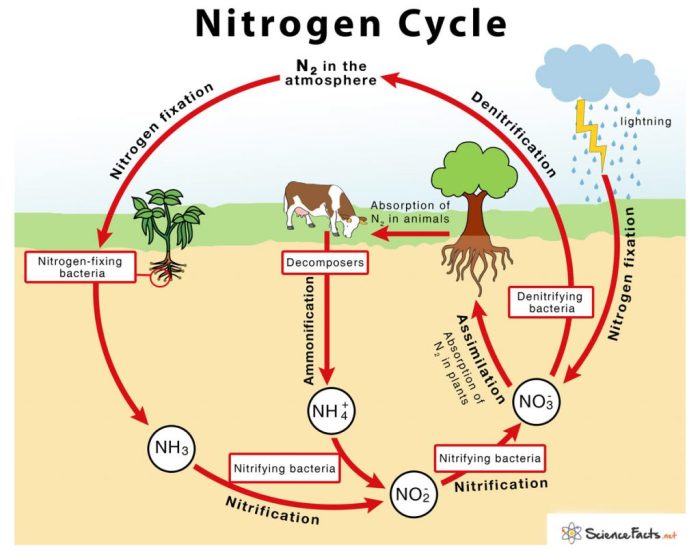
- Denitrification:Nitrate is reduced to nitrogen gas (N 2) by denitrifying bacteria, completing the cycle and returning nitrogen to the atmosphere.
Nitrogen Fixation
Nitrogen fixation is the process of converting atmospheric nitrogen into a biologically useful form, such as ammonia or nitrate. It is an essential step in the nitrogen cycle, as it makes nitrogen available to plants and other organisms. Nitrogen fixation is carried out by a variety of microorganisms, including bacteria and archaea.
There are two main types of nitrogen fixation: biological nitrogen fixation and industrial nitrogen fixation. Biological nitrogen fixation is carried out by microorganisms that have the ability to convert atmospheric nitrogen into ammonia. These microorganisms are found in a variety of habitats, including soils, oceans, and lakes.
Industrial nitrogen fixation is carried out by humans using the Haber-Bosch process. The Haber-Bosch process is a chemical process that converts atmospheric nitrogen into ammonia.
Nitrogen fixation is an important process for the nitrogen cycle. It makes nitrogen available to plants and other organisms, which use it to produce proteins and other essential molecules. Nitrogen fixation also helps to maintain the balance of nitrogen in the atmosphere.
Types of Nitrogen-Fixing Organisms
There are a variety of different nitrogen-fixing organisms, including bacteria, archaea, and cyanobacteria. Bacteria are the most common nitrogen-fixing organisms, and they can be found in a variety of habitats, including soils, oceans, and lakes. Archaea are also nitrogen-fixing organisms, and they are found in extreme environments, such as hot springs and hydrothermal vents.
Cyanobacteria are photosynthetic bacteria that can fix nitrogen. They are found in a variety of aquatic habitats, including lakes, rivers, and oceans.
| Organism | Habitat |
|---|---|
| Azotobacter | Soils |
| Clostridium | Soils |
| Rhizobium | Root nodules of legumes |
| Frankia | Root nodules of non-leguminous plants |
| Anabaena | Lakes and rivers |
| Nostoc | Soils and deserts |
Nitrification
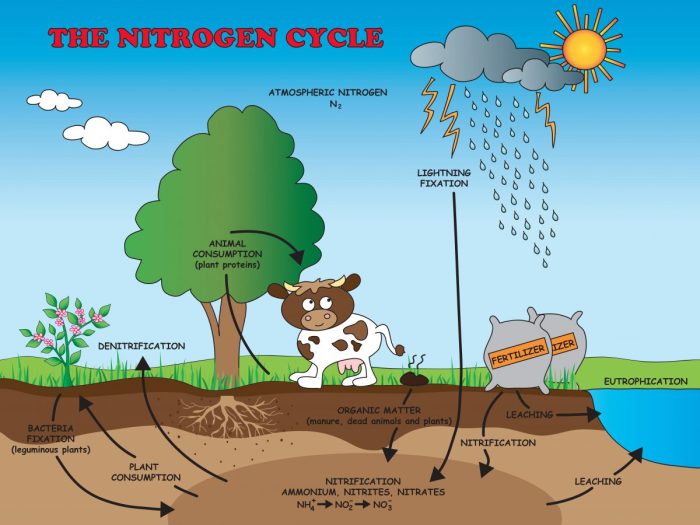
Nitrification is the biological conversion of ammonium (NH4+) to nitrate (NO3-) and nitrite (NO2-). It is a critical step in the nitrogen cycle, as it makes nitrogen available to plants and other organisms. Nitrification is carried out by a group of bacteria known as nitrifying bacteria.
Types of Nitrifying Bacteria
There are two main types of nitrifying bacteria: ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). AOB convert ammonium to nitrite, while NOB convert nitrite to nitrate. AOB include the genera Nitrosomonas and Nitrosospira, while NOB include the genera Nitrobacter and Nitrospira.
Steps of Nitrification
The process of nitrification occurs in two steps:
- Ammonia oxidation: AOB oxidize ammonium to nitrite, using oxygen as the electron acceptor.
- Nitrite oxidation: NOB oxidize nitrite to nitrate, again using oxygen as the electron acceptor.
The overall reaction for nitrification is:
NH4+ + 2O2 → NO3- + H2O + 2H+
Denitrification
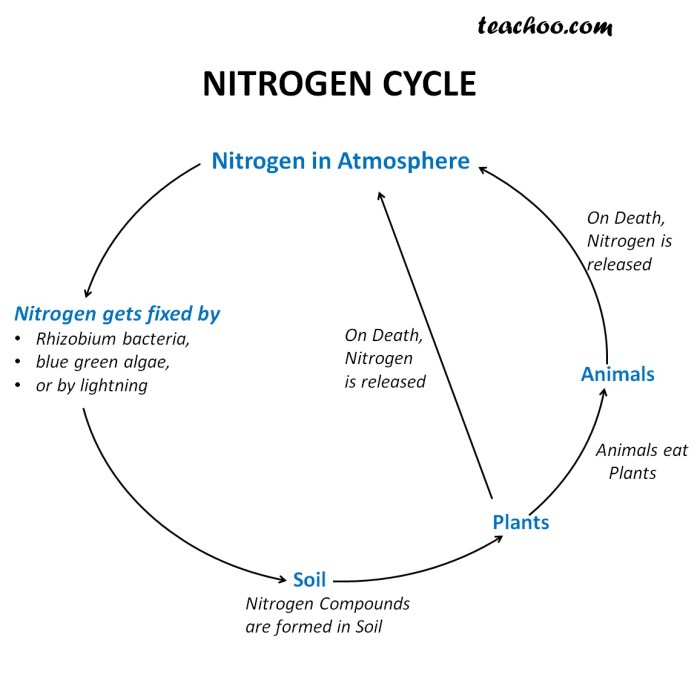
Denitrification is the final step in the nitrogen cycle, where nitrate (NO3-) and nitrite (NO2-) are converted back into atmospheric nitrogen (N2). This process is carried out by a group of bacteria known as denitrifying bacteria. Denitrification is important because it returns nitrogen to the atmosphere, making it available for plants to use.
Types of Denitrifying Bacteria
There are two main types of denitrifying bacteria: facultative and obligate. Facultative denitrifying bacteria can use either oxygen or nitrate as an electron acceptor, while obligate denitrifying bacteria can only use nitrate.
Factors Influencing Denitrification Rates
- Oxygen concentration:Denitrification rates are highest in anaerobic conditions, where there is no oxygen present.
- Nitrate concentration:Denitrification rates increase as the nitrate concentration increases.
- Temperature:Denitrification rates increase as the temperature increases.
- pH:Denitrification rates are highest at neutral pH levels.
- Organic matter:The presence of organic matter can stimulate denitrification rates.
Assimilation and Ammonification
Assimilation and ammonification are two interconnected processes that play a vital role in the nitrogen cycle. They represent the conversion of nitrogen into forms usable by plants and the return of nitrogen to the atmosphere, respectively.
-
Assimilation
Assimilation refers to the incorporation of inorganic nitrogen into organic compounds by living organisms. This process is essential for the synthesis of proteins, nucleic acids, and other nitrogen-containing molecules. Plants are the primary assimilators of nitrogen, taking up nitrate (NO3-) or ammonium (NH4+) from the soil and converting them into organic forms through the process of photosynthesis.
-
Ammonification
Ammonification is the microbial conversion of organic nitrogen compounds into ammonium (NH4+). This process occurs when microorganisms decompose organic matter, such as dead plants and animals, in the soil. The ammonium produced through ammonification can then be taken up by plants or further converted into nitrate through the process of nitrification.
The following table summarizes the key differences between assimilation and ammonification:
| Process | Definition | Role of Microorganisms |
|---|---|---|
| Assimilation | Incorporation of inorganic nitrogen into organic compounds | Limited |
| Ammonification | Conversion of organic nitrogen compounds into ammonium | Significant |
Human Impact on the Nitrogen Cycle: Diagrama Del Ciclo Del Nitrógeno
Human activities have significantly altered the nitrogen cycle, leading to potential consequences for ecosystems and human health.
One major impact is the increased production of reactive nitrogen through the Haber-Bosch process, which converts atmospheric nitrogen into ammonia for fertilizer production. This has led to a substantial increase in nitrogen availability in agricultural systems, resulting in crop yield increases but also environmental concerns.
Consequences of Human-Induced Changes
- Eutrophication:Excess nitrogen can lead to eutrophication of water bodies, causing algal blooms, oxygen depletion, and fish kills.
- Air pollution:Nitrogen oxides (NOx) emitted during combustion processes contribute to air pollution, forming smog and acid rain.
- Greenhouse gas emissions:Nitrous oxide (N2O), a potent greenhouse gas, is produced during nitrification and denitrification processes, contributing to climate change.
Mitigation Measures, Diagrama del ciclo del nitrógeno
To mitigate human impacts on the nitrogen cycle, several measures can be implemented:
- Efficient fertilizer use:Optimizing fertilizer application rates and timing to reduce nitrogen losses and minimize environmental impacts.
- Cover crops and crop rotation:Using cover crops and implementing crop rotations can improve nitrogen retention and reduce leaching.
- Precision agriculture:Utilizing technologies to monitor soil nitrogen levels and adjust fertilizer applications accordingly.
- Wastewater treatment:Implementing advanced wastewater treatment systems to remove nitrogen from wastewater before it enters water bodies.
- Engine emission controls:Reducing nitrogen oxide emissions from vehicles and industrial processes through the use of catalytic converters and other technologies.
FAQ
What is the significance of the nitrogen cycle?
The nitrogen cycle is essential for life on Earth because it ensures a continuous supply of nitrogen, a vital nutrient required for the synthesis of proteins, nucleic acids, and other biomolecules.
How does nitrogen fixation occur?
Nitrogen fixation is the process by which atmospheric nitrogen is converted into ammonia. This process is carried out by specialized microorganisms, such as bacteria and archaea, that possess the enzyme nitrogenase.
What is the role of denitrifying bacteria?
Denitrifying bacteria play a crucial role in the nitrogen cycle by converting nitrate and nitrite back into atmospheric nitrogen. This process helps to prevent the accumulation of excess nitrogen in the environment, which can have detrimental effects on ecosystems.